COVID-19 Vaccination Info
|
2021/08/10
BY ALAN YANG
TAICHUNG, MORRISON ACADEMY
|
Register for Vaccination (People Who are Qualify)
|
COVID-19 Vaccines Information
-
Moderna
-
Oxford/AstraZeneca (AZ)
-
Pfizer-BioNTech (BNT)
|
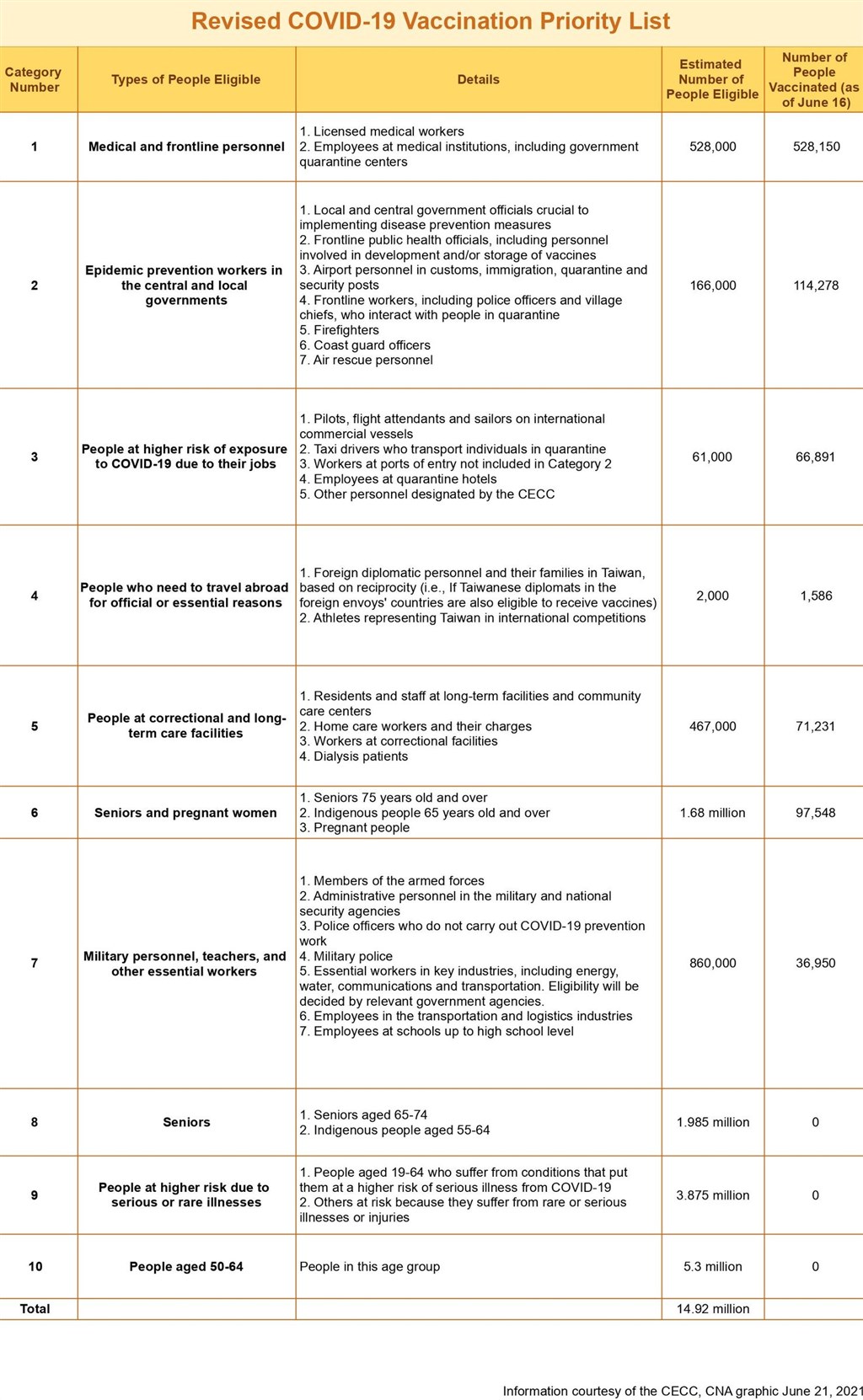
CDC Requirements for Vaccination (Priority List revised on 6/21/2021)
|
|